Thorium as Catalytic Driver
The rich advances in organoactinide catalysis that have taken place are numerous. As such, only a small selection of some notable works will be illustrated. Because of the Nobel-laureate place that metallocenes hold in the field of chemistry.
The workhorse for the catalytic actinides (uranium and thorium, to a much lesser extent, neptunium) is the five-carbon ring. As such, its origin and properties demand a brief description: its electronic and planar geometric properties are what permit it to be such a useful tool in the organometallic catalyst tool box and central to actinide catalysts.
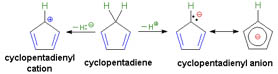
Figure: Formation of the Cp+ and Cp- species

Figure: Fully drawn Cp-
Much can be done with such a large, planar molecule that carries a formal, distributed charge of (-1).
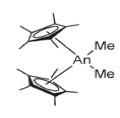
Figure: An = Th, U (adapted from Fagen et al)
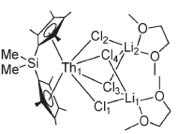
Figure: (adapted from Fagen et al)
Cp*-actinides began with the synthesis of the molecule seen in Figure 10 in 1981. The species depicted in Figure 11 came soon after and was done deliberately. As seen in 10, the angles between the thorium (An = Th) atom and the ligands could reasonably be assumed to be symmetric; this is largely accurate. The addition of the silyldimethyl (Si—(Me)2) group depicted in (11) tethered the Cp* rings, which changed the coordinating angles for the Thorium atom by about 25°, thereby offering additional access to the metal, with striking results. This change facilitated a 1000x increase in hydrogenation of 1-hexene to hexane. Later on, (10) was found to have excellent catalytic activity in the intermolecular hydroamination of terminal alkynes.
Other Catalytic Uses
Thorium is an effective catalyst for oxidizing Ammonia
Advanced Thorium Catalytic materials can be used for Petroleum Cracking Surfaces
References:
- Fagan, P.J., Manriquez, J.M., Vollmer, S.H., Day, C.S., Day, V. W., Marks, T.J. Insertion of carbon monoxide into metal-nitrogen bonds. Synthesis, chemistry, structures, and structural dynamics of bis(pentamethylcyclopentadienyl) organoactinide dialkylamides and η2-carbamoyls J. Am. Chem. Soc., 1981, 103, 2206–2220.
- Fagan, P. J., Manriquez, J. M., Vollmer, S. H., Day, C. S., Day, V. W., Marks, T. J. Insertion of carbon monoxide into metal-nitrogen bonds. Synthesis, chemistry, structures, and structural dynamics of bis(pentamethylcyclopentadienyl) organoactinide dialkylamides and η2-carbamoyls. J. Am. Chem. Soc. 103, 9, pp 2206 – 2220.
- Fendrick, C. M., Mintz, E. A., Schertz, L. D., Marks, T. J., & Day, V. W. Manipulation of organoactinide coordinative unsaturation and stereochemistry. Properties of chelating bis(polymethylcyclopentadienyl) hydrocarbyls and hydrides. Organometallics, 3(5), 819-821. 1984.
- Bruno, J.W., Smith, G.M., Marks, T.J., Fair, C. K., Schultz, A.J., Williams, J. M. Carbon-hydrogen activation mechanisms and regioselectivity in the cyclometalation reactions of bis(pentamethylcyclopentadienyl)thorium dialkyl complexes J. Am. Chem. Soc., 108, 1, 40-56, 1986.
- Straub, T., Haskel, A., Neyroud, T. G., Kapon, M., Botoshansky, M. Eisen, M.S. Intermolecular Hydroamination of Terminal Alkynes Catalyzed by Organoactinide Complexes. Scope and Mechanistic Studies. Organometallics, 20, 24, 5017-5035, 2001.


