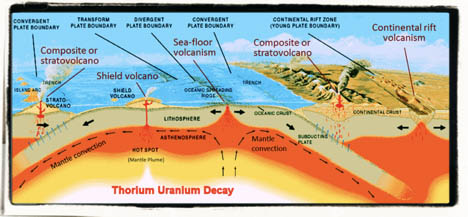
Thorium = Life on Earth – It can be said that without thorium, life on earth wouldn’t exist. The heat generated from Thorium’s natural decay is a major contributor to the internal thermal mass of Earth’s core. These radioactive isotopes generate 50% of Earth’s radiogenic heat from radioactive decay. This decay heat allows for the planets predominately iron core to remain molten and in a state of flux. This is responsible for the magnetic field that forms a protective barrier against the ravaging effects of solar winds. Without this magnetic field, Earth’s atmosphere would be blown off into the vacuum of space leaving Earth a cold lifeless rocky planet, much like the dead planet of Mercury.
The reactions in the core also create some of the chemical building blocks of life.
With Thorium helping to keep our planet able to sustain life and may hold the key to helping us maintain life on this planet, one may ask where did all this Thorium come from and how did it get here. Astrophysicists are fairly certain they know how thorium, along with the other naturally occurring heavy elements (those heavier than lead). were produced, they were born in the death of major stars. Supernovae are when massive stars have used up their fuel for nuclear fusion and collapse inward in rather astonishing fashion. The pressure, heat and gravity within the collapsed core are sufficient enough to form heavy elements in a process known as Supernova nucleosynthesis. The remaining energies force this core to explode, ejecting material into space where it is used to form new solar systems and planets.
There it remained until the first half of the 19th century when it was discovered and identified.
“Connection between life and radioactive nuclei is straightforward. No life without tectonic activity, without volcanic activity. And we know very well that geothermal energy is mostly produced by decay of uranium, thorium, and potassium.”
— Garik Israelian


